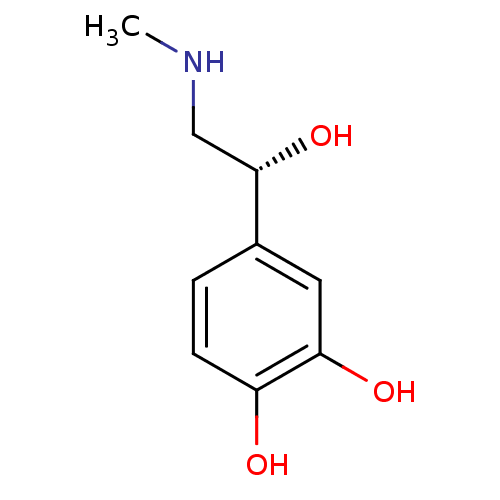
BDBM50029050 (-)-(R)-epinephrine::(-)-3,4-dihydroxy-alpha-((methylamino)methyl)benzyl alcohol::(-)-adrenaline::(R)-(-)-adrenaline::(R)-adrenaline::4-[(1R)-1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol::Adrenalin::CHEMBL679::EPINEPHRINE::adrenaline::levoepinephrine
SMILES CNC[C@H](O)c1ccc(O)c(O)c1
InChI Key InChIKey=UCTWMZQNUQWSLP-VIFPVBQESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50029050
Found 3 hits for monomerid = 50029050
TargetAlpha-2A/Alpha-2B/Alpha-2C adrenergic receptor(Homo sapiens (Human))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Binding affinity against alpha 2-Adrenergic receptor in guinea pig cerebral cortical membranes by displacement of [3H]clonidineMore data for this Ligand-Target Pair
TargetAlpha-2A/Alpha-2B/Alpha-2C adrenergic receptor(Homo sapiens (Human))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of [3H]p-aminoclonidine (PAC) binding to alpha-2 adrenergic receptor of purified human platelet plasma membranesMore data for this Ligand-Target Pair
TargetAlpha-2A/Alpha-2B/Alpha-2C adrenergic receptor(Homo sapiens (Human))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataEC50: 300nMAssay Description:Agonistic potency at alpha 2-Adrenergic receptor for inhibition of 10 uM forskolin-elicited stimulation of adenylate cyclase in human platelet membra...More data for this Ligand-Target Pair